When it comes to gases, two often misunderstood yet vital ones are carbon monoxide vs carbon dioxide. Although their names sound similar, these two gases are fundamentally different in their composition, properties, and impact on both human health and the environment. Understanding the differences between carbon monoxide and carbon dioxide is essential for ensuring safety, promoting environmental awareness, and making informed decisions regarding air quality.
In this article, we’ll explore five critical differences between carbon monoxide and carbon dioxide, covering aspects like their chemical composition, sources, effects on human health, environmental impact, and safety precautions.
1. Chemical Composition and Structure
One of the most basic and noticeable differences between carbon monoxide and carbon dioxide is their chemical composition.
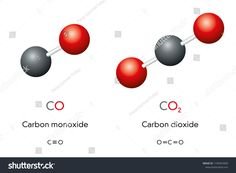
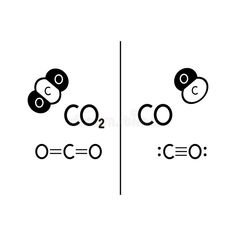
- Carbon Monoxide (CO):
Carbon monoxide is a compound made up of one carbon atom bonded to one oxygen atom. Its chemical formula is CO. Carbon monoxide has a linear molecular structure and forms a covalent bond between carbon and oxygen. - Carbon Dioxide (CO₂):
On the other hand, carbon dioxide consists of one carbon atom bonded to two oxygen atoms. Its chemical formula is CO₂, and it has a more complex structure compared to carbon monoxide. CO₂ is a linear molecule with double bonds between carbon and each oxygen atom.
This structural difference might seem small, but it significantly impacts how each gas behaves and interacts with the environment.
2. Source and Production
Both carbon monoxide and carbon dioxide occur naturally and can be man-made, but their sources differ widely.

- Carbon Monoxide (CO):
CO is mainly produced during the incomplete combustion of carbon-containing fuels like gasoline, natural gas, coal, or wood. Common sources include car exhaust, faulty gas appliances, or poorly ventilated combustion engines. The incomplete burning of fuel results in the formation of CO instead of CO₂, which is highly toxic to humans. - Carbon Dioxide (CO₂):
CO₂ is produced through the complete combustion of carbon-containing materials. It is also a natural byproduct of respiration, photosynthesis, and volcanic activity. In addition to these natural processes, CO₂ is emitted through human activities such as burning fossil fuels (coal, oil, and gas) in power plants, industrial processes, and transportation.
3. Effects on Human Health
Another significant difference between carbon monoxide and carbon dioxide is their impact on human health.

- Carbon Monoxide (CO):
Carbon monoxide is often called the “silent killer” because it is a colorless, odorless, and tasteless gas. When inhaled, CO can bind to hemoglobin in the blood, reducing the oxygen-carrying capacity of the blood. Even low levels of exposure can cause headaches, dizziness, nausea, and confusion. Prolonged exposure or high levels can lead to unconsciousness, brain damage, or death. This makes CO one of the most dangerous gases when it comes to air quality in enclosed spaces like homes or cars. - Carbon Dioxide (CO₂):
CO₂ is not inherently toxic at typical atmospheric levels. It’s naturally present in the air we breathe, making up about 0.04% of the Earth’s atmosphere. However, at high concentrations, CO₂ can displace oxygen and lead to respiratory issues, dizziness, and increased heart rate. In confined spaces, excessive CO₂ can lead to unconsciousness or suffocation. But it is generally considered less immediately dangerous than CO when it comes to health risks.
4. Environmental Impact
The environmental impacts of CO and CO₂ are also vastly different, particularly when it comes to their roles in climate change.
- Carbon Monoxide (CO):
Although CO is dangerous to human health, its direct impact on the environment is relatively minimal compared to CO₂. CO does not directly contribute to the greenhouse effect. However, it can contribute to air pollution by reacting with other compounds in the atmosphere, forming ground-level ozone, which is a component of smog. - Carbon Dioxide (CO₂):
CO₂ is a major greenhouse gas, and its accumulation in the atmosphere is one of the primary drivers of global warming and climate change. While CO₂ is naturally present in the environment, human activities like burning fossil fuels have drastically increased their levels, leading to environmental issues such as the melting of polar ice caps, rising sea levels, and more frequent extreme weather events. Reducing CO₂ emissions is a critical goal in efforts to mitigate climate change.
5. Safety Precautions
Given the distinct differences in their health impacts, the safety precautions required for CO and CO₂ vary significantly.
- Carbon Monoxide (CO):
Because of the immediate danger it poses, especially in enclosed spaces, carbon monoxide requires careful monitoring and ventilation. CO detectors are essential in homes with fuel-burning appliances, as well as garages or workplaces where CO exposure is possible. Regularly maintaining gas appliances and ensuring proper ventilation in enclosed spaces can help prevent CO buildup. If CO poisoning is suspected, immediate removal from the contaminated area and medical attention are crucial. - Carbon Dioxide (CO₂):
CO₂ safety precautions primarily focus on industrial settings or areas where high concentrations can accumulate, such as underground or poorly ventilated areas. Ensuring adequate ventilation and using CO₂ detectors in such environments can help prevent dangerous CO₂ levels. In everyday life, CO₂ poses minimal risk, though efforts to reduce CO₂ emissions are critical for long-term environmental health.
Conclusion Carbon Monoxide vs Carbon Dioxide:
While both carbon monoxide vs carbon dioxide are colorless, odorless gases, the differences between them are stark. Carbon monoxide is highly toxic to humans even at low levels and is a product of incomplete combustion, making it a silent threat in many households and industries. Carbon dioxide, on the other hand, is naturally occurring and essential for life, but its increasing concentration in the atmosphere due to human activities is driving climate change.
Understanding these differences is crucial not only for personal safety but also for addressing the larger environmental challenges of our time. Whether installing a CO detector at home or advocating for reduced CO₂ emissions, being informed about these gases can significantly impact health and the environment.





Leave a Reply